Patio NIPT Detection Kit
Genetic disease testing reagent [3], in vitro diagnostic medical device
IVD 20-127
■ Purpose of use
The Patio NIPT Detection Kit uses real-time polymerase chain reaction and fusion curve analysis of fetal cell-free DNA extracted from pregnant women's blood to help diagnose chromosomal abnormality syndrome (Down, Edward, Patau syndrome) in vitro. It is a medical device for diagnosis. (This product can be used in the case of a single fetus.)
■ Product components
1) 2X qPCR PreMix : PCR polymerase and Buffer
2) NIPT-1~12 : Primer & probe mix
3) SC DNA-1~3 : Reference control DNA
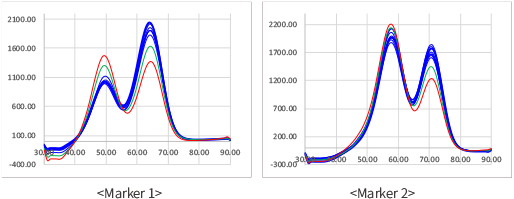
■ Result example
 ■ Storage and Validity
■ Storage and Validity
1) Store at -15℃ ~ -20℃ (both opened/unopened) and keep out of light.
2) Valid for 12 months from the manufactured date if unopened, use within 3 months after first open.


